
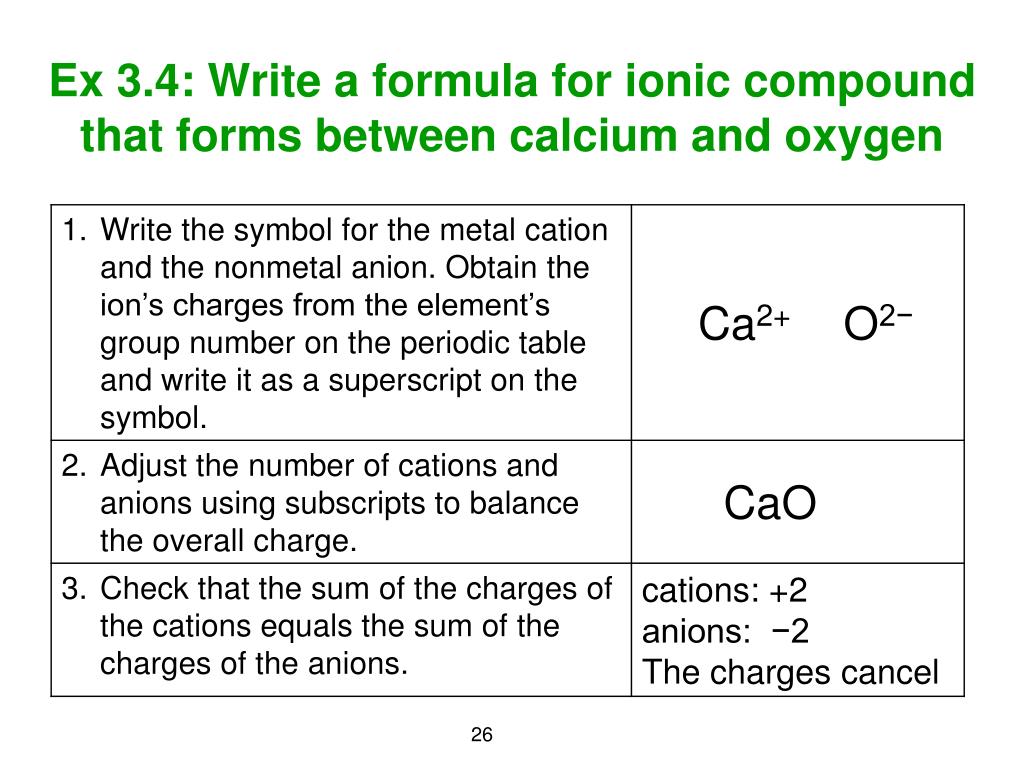
The calcium ion and the carbonate ion have a. Another role is in the chemistry of deflocculated mud, where bicarbonate ions prevent attachment of deflocculants such as lignosulfonate, onto clay edge charges. Atoms and ions are represented as spheres and are colour-coded: calcium (green), carbon (grey) and oxygen (red).Materials containing much calcium carbonate or resembling it are described as calcareous. It is a common substance found in rocks as the minerals calcite and aragonite, most notably in chalk and limestone, eggshells, gastropod shells, shellfish skeletons and pearls. A second is the formation of calcium carbonate scale on surfaces by carbonate and calcium ion reactions. Calcium carbonate is a chemical compound with the chemical formula Ca CO 3.To tell if CaCO3 (Calcium carbonate) is ionic or covalent (also called. One role is the corrosion of metals by acidic CO 2. CIBr Ionic Covalent Assign a formal charge to the 0 atom in the following molecule.Carbonates play several important roles in water mud chemistry. We further simplify it by dividing it with the common factor 2 and the chemical formula of calcium carbonate is CaCO3. The charge density of calcium ions (ions. Answer a: Answer b: Answer c: Be aware that ionic compounds are empirical formulas and so must be written as the lowest ratio of. It can be seen that the average of Ca/CO3 ratio per unit cell is largest in vaterite followed by aragonite and calcite. If no component is lost from the system (such as CO 2 gas evolving), changing pH up and down continually reverses the relative proportion of the carbonate species. SYMBOLCaCO3CHARGE+2-2After criss-crossing the symbol and charge, we get the formula as Ca2(CO3)2. Write the chemical formula for an ionic compound composed of each pair of ions. As pH rises above neutral, CO 3 –2 ions dominate. charge of one CO to form calcium carbonate (CaCO). As pH rises from acidic toward neutral, HCO 3 – ions dominate. Write the equation for the reaction of calcium ion and carbonate ion to form calcium carbonate. Carbonate chemistry involves a pH-dependent equilibrium between H 2O, H +, OH –, CO 2, HCO 3 – and CO 3 –2. You are calculating differences of numbers of equal magnitude again, and just by staring at reagents it's impossible to tell which way the pendulum is going to swing and if the result is positive or negative.An anion with formula CO 3 –2. You are gaining translational entropy from dissolving the lattice and losing translational entropy from the solvent through the inner coordination shell. On average, calcium atoms are surrounded by six oxygens from carbonate groups and one oxygen from a water molecule with about 26 of calcium atoms not associated with a water molecule. Lattice entropies are small, but entropies of solution are lower than one would think thanks to the short-range order the solvated cation imposes on the solvent. Rain or river water that come into contact with the atmosphere absorb the $\ce$. Acidic water greatly enhances the solubility of calcium carbonate, and it doesn't even need to be highly acidic. Since transition metal cations may have different oxidation states, it is best. The rock is limestone, which is usually composed of pure calcium carbonate. CO32- (Polyatomic ions have a charge even though the overall compound is neutral. Moreover, cations with a divalent charge. Cu++ may lower the growth rate of calcium carbonate.

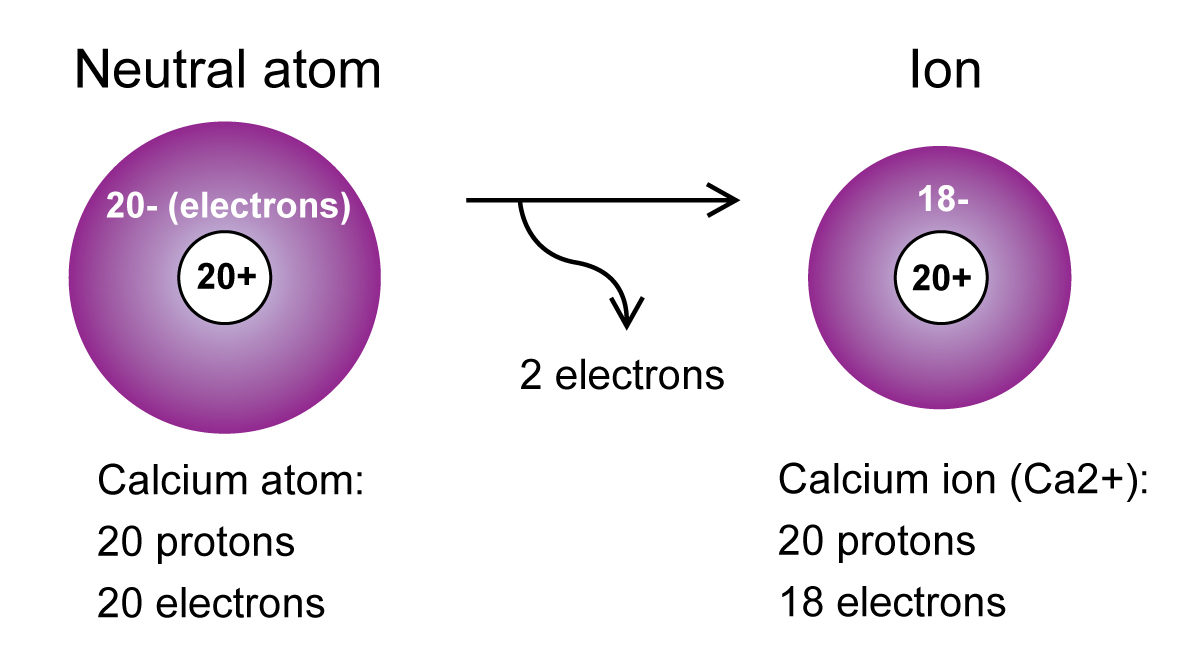
This forms because calcium carbonate dissolves. Furthermore, a recent study has demonstrated35,36 that Zn++ and. This results in a cation with 20 protons, 18 electrons, and a 2+ charge. You are probably familiar with this phenomenon: For example, a neutral calcium atom, with 20 protons and 20 electrons, readily loses two electrons.
It's just not as immediate as dissolution of the more soluble ionic compounds. Salt (halite - NaCl) is the best example.Ĭalcium carbonate, in nature, also commonly dissolves. What your teacher probably said, or didn't say but wanted to, is that some ionic compounds easily dissolve in water. First of all, the distinction between an "ionic compound" to other compounds isn't too defined.
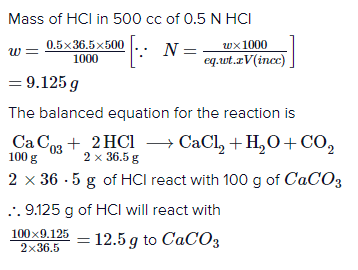
The teacher stated that the ionic compounds dissolve in water except


 0 kommentar(er)
0 kommentar(er)
